Supporting the Innovation Environment
The Institute of Hematology and Blood Transfusion has set up an internal innovation environment in cooperation with the Technology Agency of the Czech Republic (TAČR) so that it can implement a complete development cycle for modern and gene therapy drugs.
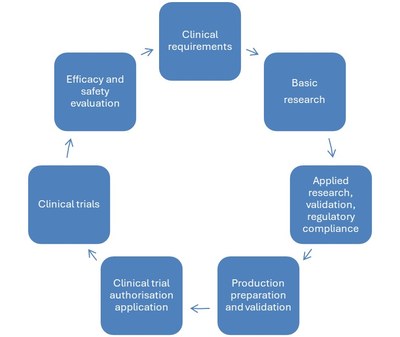
The Innovation Cycle is based on the requirements of clinicians who encounter interesting clinical problems in the care of patients at IHBT and formulate assignments to inspire basic research at IHBT.
IHBT has built an infrastructure for applied research that enables successful basic research projects to be implemented in practice - for example, to prepare the production of a new drug or to validate a new diagnostic method.
The results of applied research must be validated on patients in clinical trials. The results of the clinical trial are then fed back to clinicians and can inspire further research development.
Innovation projects of TAČR

In cooperation with the Technology Agency of the Czech Republic, IHBT implements applied research projects of the "proof-of-concept" type, which are of invaluable importance for the phase of technological transfer of basic research outputs into medical practice.
Currently, the project of the 4th public competition of TAČR SIGMA number TQ11000057 - "Successful commercialization of hematological, hemato-oncological and immunotherapeutic products II" is being implemented in this way. Within this project, which started in 2024, the following sub-projects are being implemented in turn:
|
CTT030 |
Introduction of phenotypic tests of advanced therapy medicinal products in the Good Manufacturing Practice regime |
|
CTT031 |
Transfection vectors for clinical research of BCMA/CD19 CAR-T lymphocytes in pharmaceutical quality and their accessories |
|
CTT032 |
A new molecular genetic diagnostic method for detection of partial tandem duplication in KMT2A gene in patients with myelodysplastic neoplasias |
